Imaging Analysis Laboratory
In in vivo experimental medicine, the ultimate goals of life science are the elucidation of biological functions of humans and triumph over disease. For these goals, we have been developing fundamental technologies over many years. Imaging Analysis Laboratory performs in vivo imaging using magnetic resonance imaging (MRI), micro X-ray CT, and in vivo fluorescence imaging. In vivo imaging allows noninvasive and longitudinal monitoring of changes and provides evaluation methods that address the 3Rs: Replacement, Reduction and Refinement, thus making a significant contribution to preclinical study. It can also accelerate proof-of-concept and bridging studies in preclinical studies and drug efficacy evaluations. We welcome collaborative research, contract analysis and technical advice using state-of-the-art research facilities. Please do not hesitate to contact us.
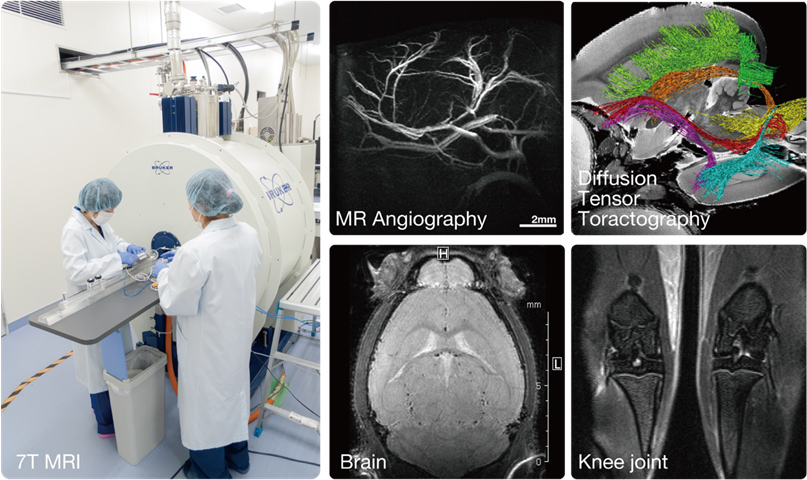
MRI and CT, with which non-invasive and repeated measurements are possible, are powerful tools for elucidating the pathophysiological mechanisms of disease model animals and evaluating of therapeutic effects. Thus, CIEM introduced the 7-Tesla MRI system and the micro X-ray CT in its collaborative research with Keio University School of Medicine. We have conducted image analyses in small laboratory animals such as mice, rats, naked mole rats, and common marmosets. Optimization of imaging procedures, development of animal beds, and careful monitoring and control of physiological states during imaging make highly reproducible measurements possible. In addition, we have taken initiatives in developing and building infrastructure of advanced MR neuroimaging techniques such as brain morphology analysis (Voxel-based morphometry; VBM)5,8 and brain function imaging (functional MRI, resting state fMRI)6. Mouse and marmoset MRI brain templates developed here is available for conducting these analysis.
MRI Templates for In vivo Mouse Brain (tissue probability map: TPM)5
C57Bl/6, BALB/cBy, C3H/He, and DBA/2 mice
http://www.nitrc.org/projects/tpm_mouse
MRI templates for In vivo Common Marmoset brain13
http://brainatlas.brain.riken.jp/marmoset/
Magnetic Resonance Imaging (MRI) 11.7T 22cm bore
In November 2023, we introduced the most advanced 11.7T 22 cm bore MRI system in Japan. As a leading MRI research institution in Japan, we are engaged in a wide range of research activities.
※References
-
Multimodal analyses of a non-human primate model harboring mutant amyloid precursor protein transgenes driven by the human EF1α promoter.
Yoshimatsu S*, Seki F*, Okahara J*, Watanabe H, Sasaguri H, Haga Y, Hata J, Sanosaka T, Inoue T, Mineshige T, Lee CY, Shinohara H, Kurotaki Y, Komaki Y, Kishi N, Murayama AY, Nagai Y, Minamimoto T, Yamamoto M, Nakajima M, Zhou Z, Nemoto A, Sato T, Ikeuchi T, Sahara N, Morimoto S, Shiozawa S, Saido TC, Sasaki E, Okano H. (*equal contribution)
Neurosci Res. 2022 Dec ; 185:49-61.
-
Effects of chronic caffeine intake and withdrawal on neural activity assessed via resting-state functional magnetic resonance imaging in mice.
Rikitake M, Notake S, Kurokawa K, Hata J, Seki F, Komaki Y, Oshiro H, Kawaguchi N, Haga Y, Yoshimaru D, Ito K, Okano HJ.
Heliyon. 2022 Nov 19;8(11):e11714.
-
Comprehensive Volumetric Analysis of Mecp2-Null Mouse Model for Rett Syndrome by T2-Weighted 3D Magnetic Resonance Imaging.
Akaba Y*, Shiohama T*, Komaki Y*, Seki F, Ortug A, Sawada D, Uchida W, Kamagata K, Shimoji K, Aoki S, Takahashi S, Suzuki T, Natsume J, Takahashi E, Tsujimura K. (*equal contribution)
Front Neurosci. 2022 May 10;16:885335.
-
Differential effects of aquaporin-4 channel inhibition on BOLD fMRI and diffusion fMRI responses in mouse visual cortex.
Komaki Y, Debacker C, Djemai B, Ciobanu L, Tsurugizawa T, Le Bihan D.
PLoS One. 2020;15(5):e0228759.
-
Quantitative temporal changes in DTI values coupled with histological properties in cuprizone-induced demyelination and remyelination.
Yano R, Hata J, Abe Y, Seki F, Yoshida K, Komaki Y, Okano H, Tanaka KF.
Neurochem Int. 2018 Oct;119:151-158.
-
Mapping orbitofrontal-limbic maturation in non-human primates: A longitudinal magnetic resonance imaging study.
Uematsu A, Hata J, Komaki Y, Seki F, Yamada C, Okahara N, Kurotaki Y, Sasaki E, Okano H.
Neuroimage. 2017 Dec;163:55-67.
-
Developmental trajectories of macroanatomical structures in common marmoset brain.
Seki F, Hikishima K, Komaki Y, Hata J, Uematsu A, Okahara N, Yamamoto M, Shinohara H, Sasaki E, Okano H.
Neuroscience. 2017 Nov 19;364:143-156.
-
In vivo microscopic voxel-based morphometry with a brain template to characterize strain-specific structures in the mouse brain.
Hikishima K, Komaki Y, Seki F, Ohnishi Y, Okano HJ, Okano H.
Sci Rep. 2017 Mar 7;7(1):85.
-
Functional brain mapping using specific sensory-circuit stimulation and a theoretical graph network analysis in mice with neuropathic allodynia.
Komaki Y, Hikishima K, Shibata S, Konomi T, Seki F, Yamada M, Miyasaka N, Fujiyoshi K, Okano HJ, Nakamura M, Okano H.
Sci Rep. 2016 Nov 29;6:37802.
-
Chronic multiscale imaging of neuronal activity in the awake common marmoset.
Yamada Y, Matsumoto Y, Okahara N, Mikoshiba K.
Sci Rep. 2016 Oct 27;6:35722.
-
Voxel-based morphometry of the marmoset brain: In vivo detection of volume loss in the substantia nigra of the MPTP-treated Parkinson's disease model.
Hikishima K, Ando K, Komaki Y, Kawai K, Yano R, Inoue T, Itoh T, Yamada M, Momoshima S, Okano HJ, Okano H.
Neuroscience. 2015 Aug 6;300:585-92.
-
Parkinson Disease: Diffusion MR Imaging to Detect Nigrostriatal Pathway Loss in a Marmoset Model Treated with 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Hikishima K, Ando K, Yano R, Kawai K, Komaki Y, Inoue T, Itoh T, Yamada M, Momoshima S, Okano HJ, Okano H.
Radiology. 2015 May;275(2):430-7.
-
Multidimensional MRI-CT atlas of the naked mole-rat brain (Heterocephalus glaber).
Seki F, Hikishima K, Nambu S, Okanoya K, Okano HJ, Sasaki E, Miura K, Okano H.
Front Neuroanat. 2013 Dec 20;7:45.
-
In vivo tracing of neural tracts in tiptoe walking Yoshimura mice by diffusion tensor tractography.
Takano M, Komaki Y, Hikishima K, Konomi T, Fujiyoshi K, Tsuji O, Toyama Y, Okano H, Nakamura M.
Spine (Phila Pa 1976). 2013 Jan 15;38(2):E66-72.
-
Quantitative comparison of novel GCaMP-type genetically encoded Ca(2+) indicators in mammalian neurons.
Yamada Y, Mikoshiba K.
Front Cell Neurosci. 2012 Oct 8;6:41.
-
Population-averaged standard template brain atlas for the common marmoset (Callithrix jacchus).
Hikishima K, Quallo MM, Komaki Y, Yamada M, Kawai K, Momoshima S, Okano HJ, Sasaki E, Tamaoki N, Lemon RN, Iriki A, Okano H.
Neuroimage. 2011 Feb 14;54(4):2741-9.
-
Diffusion-tensor neuronal fiber tractography and manganese-enhanced MR imaging of primate visual pathway in the common marmoset: preliminary results.
Yamada M, Momoshima S, Masutani Y, Fujiyoshi K, Abe O, Nakamura M, Aoki S, Tamaoki N, Okano H.
Radiology. 2008 Dec;249(3):855-64.
-
In vivo tracing of neural tracts in the intact and injured spinal cord of marmosets by diffusion tensor tractography.
Fujiyoshi K, Yamada M, Nakamura M, Yamane J, Katoh H, Kitamura K, Kawai K, Okada S, Momoshima S, Toyama Y, Okano H.
J Neurosci. 2007 Oct 31;27(44):11991-8.